bromine valence electrons|What Are Valence Electrons? Definition and Periodic : Tagatay 119 rows — Mar 23, 2023 — Find the valence electrons of bromine and other . WhoScored.com provides live football scores and detailed statistics for fans worldwide.
PH0 · What Are Valence Electrons? Definition and Periodic
PH1 · Valence Electrons Chart for All Elements
PH2 · Khan Academy
PH3 · How to Find the Valence Electrons for Bromine (Br)
PH4 · How many valence electrons does bromine have?
PH5 · How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bro
PH6 · How Many Valence Electrons Does Bromine (Br) Have?
PH7 · How Many Valence Electrons Does Bromine (Br)
PH8 · Bromine Electron Configuration (Br) with Orbital Diagram
PH9 · Bromine
At $5 minimum deposit casino NZ sites, you can play all the game types with a small deposit. CasinoAlpha selected only the best $5 deposit casino NZ sites which offer a wide array of games for you to enjoy without breaking the bank: A variety of slots, including high-RTP pokies and progressive jackpots;
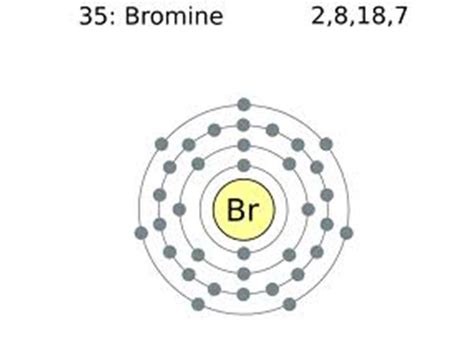
bromine valence electrons*******Set 2, 2020 — Learn two methods to determine the number of valence electrons in Bromine (Br) using the Periodic Table and the electron configuration. Watch a helpful video with examples and tips for drawing Lewis structures.bromine valence electrons What Are Valence Electrons? Definition and Periodic 119 rows — Mar 23, 2023 — Find the valence electrons of bromine and other .Peb 3, 2021 — Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. The valence electrons of bromine are seven and the valency is one, based on its electron configuration and periodic .Dis 15, 2019 — If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org .Bromine is a volatile red-brown liquid with the symbol Br and atomic number 35. It belongs to the halogen group and has seven valence electrons. Learn about its history, isotopes, physical and chemical properties, and applications.Mar 3, 2021 — Learn what valence electrons are and how to find them for any element. Bromine has seven valence electrons in the 4s and 4p shells, according to the web page.Learn how to identify and count valence electrons, the electrons in the outermost shell of an atom, with examples and diagrams. See how valence electrons affect chemical reactions and .Peb 1, 2021 — In this article today we are going to tell you about the electron configuration of Bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. Please read the full article below for more .Bromine is a chemical element; it has symbol Br and atomic number 35. . [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full .How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can help you determine the number of electrons in the outermost shell of any atom. You will also learn why valence electrons are important for chemical bonding and reactivity.Peb 3, 2021 — Step 4: Find Valence Electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² .May 19, 2024 — This table of element valences includes the maximum valence and most common valence values in chemistry. . You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking . Bromine-1, +1, (+3), (+4), +5: 36: Krypton: 0: 37: Rubidium +1: 38 .Abr 17, 2023 — Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For the main group elements (groups designated with a .Peb 1, 2021 — Bromine Valence Electrons. Valence electrons can be found in the p and S highest energy orbitals. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The valence electrons are there in the 4s and 4p orbitals which gives Bromine 7 valence electrons. See the picture below;May 6, 2018 — 7 only the electrons in the outmost shell are valance electrons.All but seven of the electrons in bromine are in lower shells Bromine is in family VII A. the same as Fluorine Chlorine. All members of the family have seven valance electron hence the name 7A.Abr 17, 2023 — The Modern Periodic Table and Chemical Bonds. As described in Section 10.6, the modern periodic table is arranged based on an atom's valence electrons.But what does this tell us about how they form chemical bonds with each other? Why do sodium atoms and chlorine atoms combine in a 1:1 ratio, while sodium and oxygen atoms combine in a 2:1 ratio?There is only one element in bromine molecule. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of bromine atoms. valence electrons given by bromine atoms = 7 * 2 = 14; Total valence electrons = 14Set 20, 2022 — The halogens all have the general electron configuration \(ns^2np^5\), giving them seven valence electrons. They are one electron short of having the full outer \(s\) and \(p\) sublevel, which makes them very reactive. . while bromine is a liquid, and iodine is a solid. Review. Pick two elements that are halogens. For each, write the name .
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.bromine valence electronsElement Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .What Are Valence Electrons? Definition and Periodic Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine has thirty-five protons and forty-four neutrons in its nucleus, and thirty-five electrons in four shells. It is located in group seventeen, period four and block p of the periodic .
Hul 20, 2023 — A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. An atom may give, take, or share electrons with another atom to achieve a full valence shell, the most stable electron configuration.
Hun 30, 2023 — Because the halogen elements have seven valence electrons, they only require one additional electron to form a full octet. This characteristic makes them more reactive than other non-metal groups. . Bromine - Bromine has an atomic number of 35 with a symbol of Br. It was first discovered in 1826. In its elemental form, .Peb 1, 2021 — Get a thorough understanding of Bromine valence electrons here in the article. You will further explore the elements with its others significant properties. In chemistry, Bromine is a chemical element with its atomic number 35.Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .How do you find the formula of an ionic compound from its name or vice versa? Watch this worked example video to learn how to apply the rules of naming and writing ionic formulas. Khan Academy offers free, world-class education for anyone, anywhere.
Knowing how to say “sorry” in Korean can help you overcome awkward and embarrassing situations. Using the right phrase to say ‘sorry’ is essential to deal with the situation. In Korean, there are several ways to say “I’m sorry.” We’ve written them in the Korean alphabet and romanization below.
bromine valence electrons|What Are Valence Electrons? Definition and Periodic